Background:
Personalized prophylaxis with factor VIII (FVIII) concentrates remains the mainstay therapy for patients with severe hemophilia A. The bispecific monoclonal antibody emicizumab, which mimics FVIII function, is also approved for prophylaxis in hemophilia A patients. Head-to-head comparative studies on the relative efficacy of different therapeutic strategies are not feasible in a rare disease such as hemophilia A. In such situations, the use of indirect comparison methods can help to compare relative efficacies. Matching-adjusted indirect comparison (MAIC) is a well-established statistical method to indirectly compare outcomes from separate clinical studies. It can be used to adjust for differences in patient characteristics between studies, with the goal to reduce bias in the treatment effect estimates which can occur with comparison of efficacy data across clinical trials.
Aims:
To indirectly compare the efficacy of a pharmacokinetics (PK)-guided personalized prophylaxis regimen with a recombinant, fourth generation, FVIII concentrate, simoctocog alfa (Nuwiq ®), against standard prophylaxis with the bispecific monoclonal antibody emicizumab (Hemlibra ®) in patients with hemophilia A using MAIC methodology.
Methods:
After matching study populations, accounting for clinically important baseline characteristics,individual patient-level data (IPD) from 65 patients treated with simoctocog alfa from the NuPreviq study were compared against aggregate data for emicizumab administered once weekly (Q1W, N=99), every 2 weeks (Q2W, N=35) or every 4 weeks (Q4W, N=41) from the HAVEN 3 and HAVEN 4 studies. All patients enrolled in all studies were male.
Baseline patient variables for matching were chosen based on availability, clinical relevance and distribution overlap. Baseline age and body weight were used to re-weight the simoctocog alfa IPD to match the aggregate data reported for the emicizumab studies. Patients were removed from the NuPreviq study population if they did not meet the eligibility criteria for the comparator studies (but none were excluded from this analysis based on this). Unanchored indirect treatment comparisons were performed for various clinical outcomes using the re-weighted data for simoctocog alfa and the published comparator study data. The endpoints analyzed were total annualized bleeding rates (ABRs) and percentage of patients with zero bleeds. All endpoints considered treated and untreated bleeds.
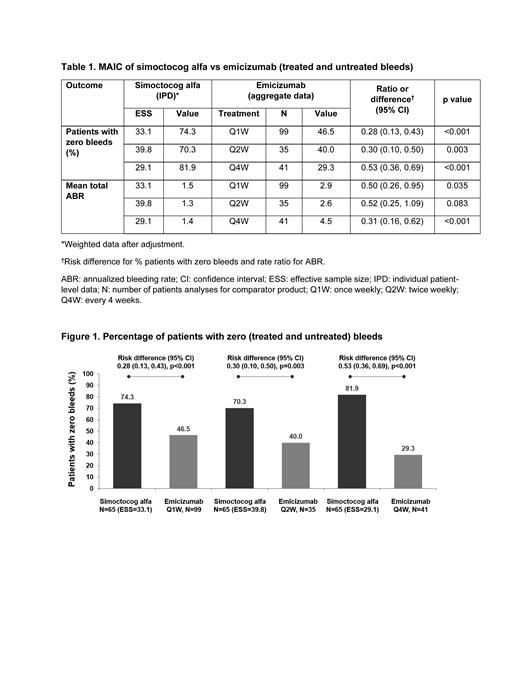
Results:
No patients were excluded from the NuPreviq study population due to imposing the body weight or age criteria of the HAVEN 3 and 4 studies. After matching populations, the effective sample size for simoctocog alfa was 33.1, 39.8 and 29.1 participants for the Q1W, Q2W and Q4W comparisons, respectively. The percentage of patients with zero bleeds was significantly higher with simoctocog alfa across all 3 comparisons: simoctocog alfa (74.3%) vs emicizumab Q1W (46.5%); simoctocog alfa (70.3%) vs emicizumab Q2W (40.0%), and simoctocog alfa (81.9%) vs emicizumab Q4W (29.3%; Figure 1, Table 1). The total ABRs were significantly lower with simoctocog alfa versus emicizumab Q1W (1.5 vs 2.9) and emicizumab Q4W (1.4 vs 4.5; Table 1). When comparing simoctocog alfa versus emicizumab Q2W, the difference was not statistically significant (1.3 vs 2.6; Table 1).
Conclusion:
Indirect comparisons demonstrated that PK-guided, personalized prophylaxis with simoctocog alfa can lead to statistically significantly higher zero bleed rates and decreased ABRs compared with standard emicizumab prophylaxis. This MAIC analysis provides important comparative efficacy and utilization data, which can help guide patients and physicians in making decisions regarding product choice for prophylaxis regimens.
Disclosures
Kessler:Genentech: Other: Scientific advisory board; CSL Behring: Other: Scientific advisory board; Bayer: Consultancy, Other: Chair, DSMB, scientific advisory board, Research Funding; Novo Nordisk: Other: Scientific advisory board; Octapharma: Other: Scientific advisory board, Research Funding. Corrales-Medina:Octapharma: Other: Scientific advisory board; Genentech: Other: Scientific advisory board; CSL Behring: Other: Scientific advisory board; Bayer: Other: Scientific advisory board, Research Funding; Takeda: Other: Scientific advisory board. Mannucci:Bayer: Honoraria, Other: Scientific advisory board, Speakers Bureau; Grifols: Honoraria, Speakers Bureau; Kedrion: Honoraria, Other: Scientific advisory board, Speakers Bureau; Octapharma: Honoraria, Other: Scientific advisory board, Speakers Bureau; Roche: Other: Scientific advisory board; Takeda: Other: Scientific advisory board; Werfen: Other: Scientific advisory board. Tarantino:Takeda: Other: Clinical trial investigator, Research Funding; Amgen: Consultancy; Biomarin: Consultancy; Genentech: Consultancy; Novartis: Consultancy; Octapharma: Consultancy, Other: Clinical trial investigator; Principia: Consultancy; Spark: Other: Clinical trial investigator.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal